The glass ceiling of industrial biotech
I discovered mRNA vaccines around 2020. The technology was almost science-fiction, and made me realize how far we had advanced in our understanding of genetics and cellular mechanisms. This drove me to dig into the field of synthetic biology, which seemed like an obvious way to maximise raw materials availability, and synthesise almost anything on demand: just program a microorganism with DNA, grow it, and profit.
Reality is tougher. Bioproduction has clear wins (vitamins, amino acids, drugs, antibodies, ethanol…), but given the magnitude of the promise, we should expect more.
For years, the field leaned on high‑throughput screening: build and test thousands of strains and hope the best rise to the top. It was supposed to revolutionize industrial biotech and unlock hundreds of product‑ready strains.
That revolution stalled. The design space is simply too vast. Even informed search demands millions of experiments, keeping costs high. And finding a promising strain is only the beginning, scale‑up adds costs and uncertainty.
Fortunately, this is changing. Instead of brute‑force high‑throughput, we can now optimize for predictive throughput. The goal shifts from “more experiments” to higher success rates not just at bench scale but through pilot and into the plant. Test just 100 strains and see 10+ with industrial potential.
Genome-to-factory AI model
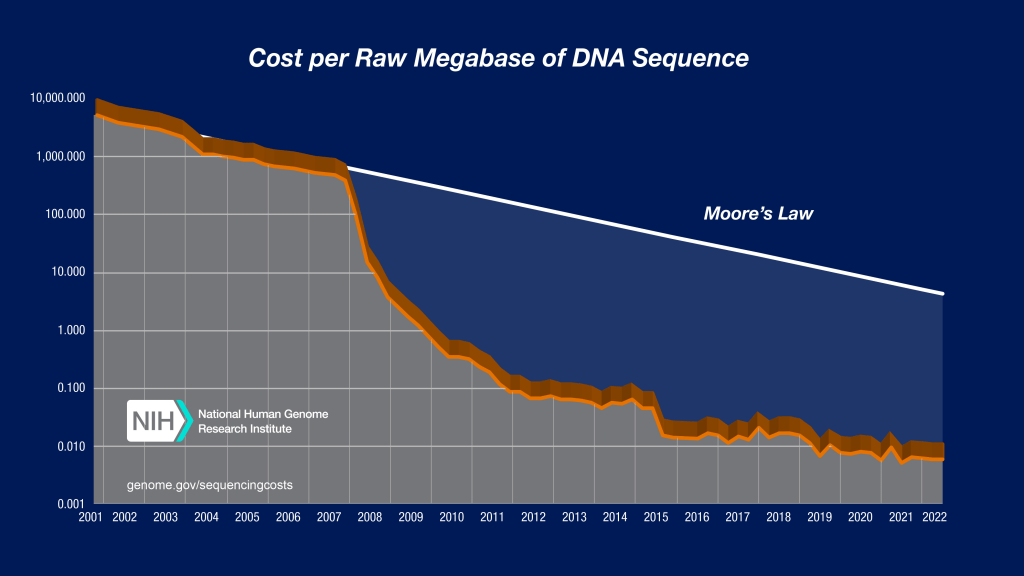
Acquiring biological data has never been easier or cheaper. Advances in DNA sequencing and synthesis have driven down the cost of reading and writing genetic information dramatically, faster than an inverted Moore’s Law.
New AI models unlock the full value of this data. DNA LLMs coupled with scale‑up models trained on production metrics can materially increase the success rate of strain design.
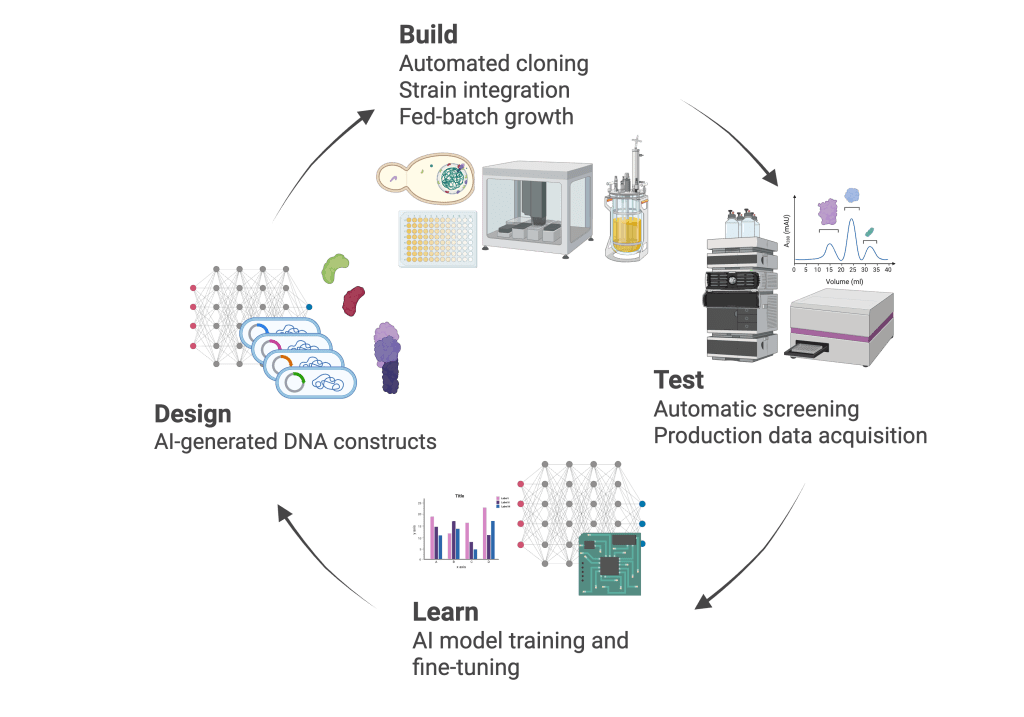
The traditional DBTL loop becomes an autonomous biofoundry:
- Design: DNA LLM proposes new DNA sequences, which are scored for industrial potential
- Build: Liquid‑handling robots convert the best designs to physical strains, and grow them under production‑like conditions
- Test: Analytical systems assess performance, streaming real‑time production data.
- Learn: Results flow back as training data and immediately generate the next wave of designs
Because pretrained DNA LLMs are available and public datasets are abundant, training sets have to be meaningful, but not necessarily massive to yield precise predictions.
With inexpensive data collection, startups can now collect their own data, and train their own AI models. The data flywheels, and the data-driven moat it creates, is finally within reach for new entrants.
Full-Stack Bioproduction
A superior strain is only half the battle: the real prize is consistent, profitable manufacturing.
Historically, high data costs and heavy CapEx requirements forced startups to position themselves as service providers: customers owned the data and the plants, and startups often stopped at proof‑of‑concept.
Today, advances in bioreactors, expanded fermentation capacity, and better scale‑up models are narrowing the gap between lab and factory.
Training AI models directly on proprietary, industrially realistic data closes it completely, making strain development cheaper, faster, and less risky. Standardized bioprocesses for fermentation and downstream processing enable a full-stack bioproduction platform.
AI-powered bioproduction for industrial ingredients
Proprietary data collection, AI-powered predictive throughput, and full-stack bioproduction: those are the three fundamental pillars of our strategy at baCta.
This integrated, AI‑powered platform isn’t just a theory. We’ve validated it with our first product, astaxanthin, the most potent antioxydant available today. Each new ingredient compounds the cycle: better data → better predictions → faster design of scalable strains → faster industrial production.
We deeply believe microorganisms are programmable molecular factories capable of synthesizing almost anything. The potential of bioproduction is about to be realized, and we’re building it.

